SCIENCE OF CERTAINTY
Progress your preclinical
drug development
with speed and certainty
Highly Specialized Preclinical Solutions
CRO Services
Swipe to see all services
Liver (MASH)
Explore our industry-leading preclinical MSH model portfolio. Our GAN DIO-MASH mouse model is ranked #1 by the LITMUS consortium for human translatability. Profile your drug candidate in our biopsy-confirmed mouse models of MASH, using clinical endpoints and AI-assisted histological analyses.
Obesity
We have more than 15 years experience within preclinical obesity studies to help progress your drug candidate. We provide off-the-shelf diet-induced obese (DIO) rodent models for profiling the efficacy and mode of action of your compound on endpoints ranging from food intake over energy expenditure to whole-brain activation signatures.
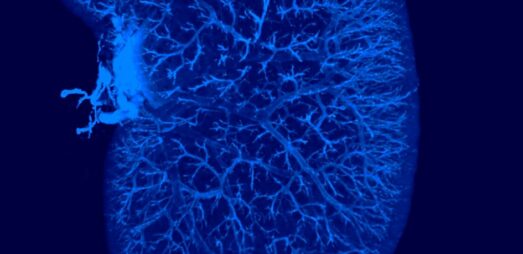
Kidney (CKD/DKD)
Progress your drug discovery with Gubra’s broad portfolio of mouse models recapitulating acute and chronic kidney diseases. Our ReninAAV hypertension-accelerated diabetic kidney disease (DKD) mouse is one of the most advanced models available for profiling drug candidates with therapeutic potential in DKD.
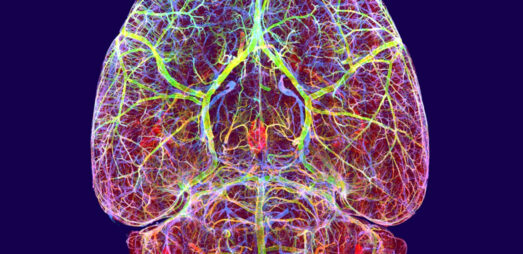
CNS
Gubra’s unique 3D light sheet imaging platform can visualize drug distribution, map drug targets and quantify therapeutic responses in the intact brain at single cell resolution. Our fully automated light sheet imaging pipeline accommodates up to 100 brains per study with a fast turn-around time of 2.5 months. Get instant access to your imaging data in our interactive atlas-guided 3D brain-viewer.
Lung (IPF)
We provide end-to-end customized studies in our spirometry-confirmed and bleomycin-induced mouse models of idiopathic pulmonary fibrosis (IPF). Accelerate your drug candidate by exploring pivotal lung functional endpoints in combination with AI-assisted histopathological scoring and quantitative histology.
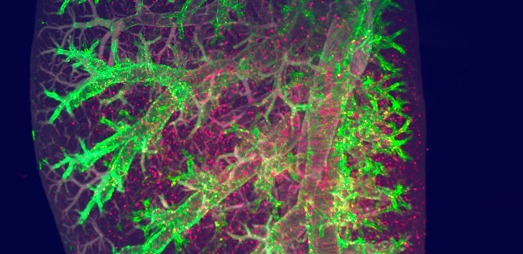
Diabetes
We are experts in preclinical diabetes research, and offer customized studies in canonical rodent models of type 2 and type 1 diabetes to guide development of your anti-diabetic compound. Study data provided by our multidisciplinary teams can inform about efficacy on metabolic and glycemic endpoints as well as whole-pancreas islet and β-cell numbers for interpreting mode of action.
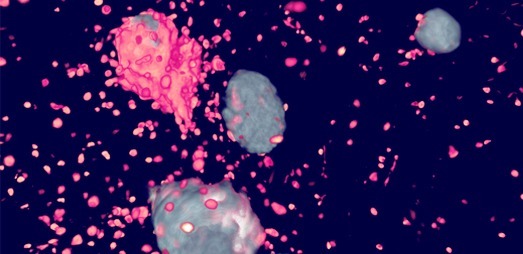
3D imaging
Gubra’s light-sheet microscopy platform allows for 3D imaging of all organs in the mouse and rat. Our fully automated imaging pipeline works seamlessly in combination with our in vivo models and in-house developed AI-driven image analysis to ensure precision-mapping of drug distribution and quantification of histological endpoints.

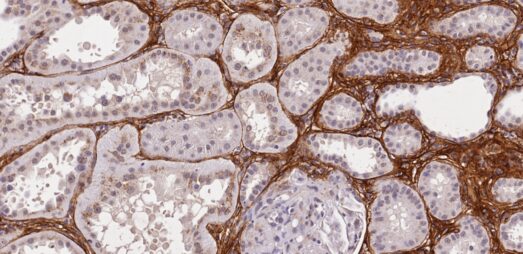
Histology
High-quality histology is essential in preclinical drug development and can inform about drug mode of action. We offer routine and advanced histology services, including core histology, multiplex immunohistochemistry and RNAScope® as well unbiased stereology analysis for best-practice whole-sample quantitative histology.
RNAseq
We offer a full palette of advanced sequencing technologies, including bulk RNAseq, single-nucleus RNAseq, spatial transcriptomics and ATACseq. We accept most types of tissues, including fresh frozen or archival samples (FFPE). Trust Gubra for fast turnaround and expert bioinformatics. Get instant access to your data in GubraView, our interactive data viewer.
target and peptide drug discovery
Discovery, Development & Partnerships
Swipe for more
Pipeline
Background
With current weight loss treatments, 20–40% of body weight reduction comes from unwanted loss of lean mass (muscles, bones, internal organs). Gubra’s UCN2 program is developed for high quality weight loss. In preclinical models UCN2 prevented incretin induced lean mass loos while enhancing fat mass reduction.
Compound
Urocortin-2 analogue and a potent and highly selective Corticotropin-releasing hormone receptor 2 (CRHR2) agonist that has been designed for weekly dosing.
Internal
Background
Narcolepsy type 1 is characterized by the loss of hypothalamic neurons producing orexin (hypocretin), a neuropeptide critical for maintaining wakefulness. Orexin receptor agonists activate orexin receptors (OX1R/OX2R), compensating for the deficiency. This mechanism directly targets the underlying pathophysiology, offering a therapeutic potential to improve clinical symptoms such as excessive daytime sleepiness and cataplexy.
Compound
A novel, peptide-based dual orexin receptor agonist designed to restore the underlying orexin deficiency and reestablish normal wakefulness regulation.
Internal
Background
Injectable peptide-based GLP-1 receptor (GLP1R) agonists have shown remarkable therapeutic success in the treatment of type2 diabetes and obesity, as a monotherapy or in combinations.
Compound
Potent and selective GLP-1 receptor agonist from secretin backbone with good formulation properties excellent in vivo efficacy and PK properties.
Internal
Background
Hypoparathyroidism is a rare endocrine disorder in which the body produces insufficient levels of parathyroid hormone (PTH). PTH is crucial for regulating and maintaining a balance of calcium and phosphorus in the body. Low PTH levels lead to hypocalcemia and hyperphosphatemia, which can lead to muscle cramps, tingling sensations, and in severe cases, seizures or cardiac issues.
Compound
A novel, peptide-based PTH receptor agonist designed to restore calcium and phosphate levels in patients suffering from hypoparathyroidism.
Internal
Background
Despite significant advancements with approved anti-obesity medications, a substantial medical need persists. Current therapies, while effective for many, often fall short in delivering sustained, long-term and muscle preserving weight loss.
Compound
Undisclosed
Internal
Background
AbbVie and Gubra announced a license agreement to develop an amylin analog for the treatment of obesity. The agreement enables the incorporation of GUB014295, an amylin analogue discovered and developed by Gubra, into AbbVie's global infrastructure (ABBV-295). Amylin is a naturally occurring hormone secreted by pancreatic beta cells that plays a crucial role in energy balance and glucose homeostasis. As a satiety signal, it activates pathways in the brain to suppress appetite and reduce food intake.
Compound
A long-acting amylin analog.
Partner
Clinical
The Phase 1 clinical trial is a two-part, single center, double-blind (within cohorts), randomized, placebo-controlled, single (Part 1) and multiple (Part 2) ascending subcutaneous dose study of GUB014295. Part 1 has been completed; Part 2 is ongoing. More information on this trial can be found at https://www.clinicaltrials.gov/ (NCT: 06144684).
Background
BI 1820237, which is fully outlicensed to Boehringer Ingelheim, is a long-acting neuropeptide Y receptor type 2 (NPY2R) agonist. In October 2024, Boehringer Ingelheim discontinued its development for obesity. Potential opportunities outside the obesity indication are now being explored by Boehringer Ingelheim.
Compound
Undisclosed
Partner
Background
BI 3034701, fully outlicensed to Boehringer Ingelheim, is a triple agonist peptide.
Compound
A long-acting triple agonist peptide with a potential to become a next-generation and first-in-class obesity treatment.
Partner
Clinical
The Phase 1, first-in-human, randomized, placebo-controlled trial, will assess safety, tolerability, and pharmacokinetics of the drug candidate (BI 3034701). The trial has 2 parts. Part A is open to healthy men between 18 and 55 years of age. Part B is open to people between 18 and 65 years of age with overweight or obesity who are otherwise healthy.
Background
Building on a longstanding partnership, Boehringer Ingelheim and Gubra continue their collaborative efforts to identify and validate novel peptide-based therapies for obesity. Leveraging complementary strengths in Research and Development , the partnership aims to deliver innovative peptide treatments to improve health outcomes for people living with obesity.
Compound
Undisclosed
Partner
Background
Further building on their history of successful partnership, Gubra and Boehringer Ingelheim have entered a collaboration taking a new approach to discover novel peptides to deliver innovative treatments with the aim of improving health outcomes for people living with obesity.
Compound
Undisclosed
Partner
Background
Collaboration with Amylyx Pharmaceuticals to develop a novel long-acting GLP-1 Receptor Antagonist.
Compound
Undisclosed
Partner
Background
Undisclosed
Compound
Undisclosed
Partner
Drug Discovery & Development
Gubra has a long history of successful peptide drug discovery. Our AI-driven streaMLine platform quickly develops a peptide hit into a clinical ready drug candidate with balanced properties and strong IP protection. We do linear peptides, cyclic peptides, disulfide-rich peptides (DRP), peptide drug-conjugates and more.

Partnering
Gubra is known as a professional and scientific strong collaboration partner, and we have several successful partnerships ongoing. Our agile and data-driven approach to project management is valued by our partners and is key to our success and speed. Partnerships are important to us, and we always look for new collaboration opportunities.

Science with purpose
About us
CRO & Biotech Company
Gubra is specialized in high-end preclinical contract research and peptide-based drug discovery within metabolic and fibrotic diseases. The combination of our advanced, high-end models and technologies ensures rapid turnaround time and high quality data.
Workplace full of opportunities
We offer an exciting and challenging work environment, where job satisfaction and personal development are top priorities. Together, we are scientific entrepreneurs. Explore our open positions if you would like to join the Gubra team.
We are scientific entrepreneurs
Investors
Investor Relations
We are committed to providing our shareholders and the media with reliable and transparent information about our business, discovery & development programs and results in an open and timely manner.
Gubra announces deal with AbbVie amounting to more than $ 2.2 billion
The agreement enables AbbVie to develop GUBamy, our in-house developed, best-in-class, long-acting amylin analog in development for weight management. Gubra will receive $350 million in total upfront payment and will be eligible to receive up to $1.875 billion in development, commercial and sales milestone payments with tiered royalties on global net sales.
We act responsibly
Sustainability
For further information
Contact us
Gubra
Hørsholm Kongevej 11B
2970 Hørsholm
Denmark
+45 3152 2650


